

He considered this ‘the most economic of all physical laws’. Nicholas Georgescu-Roegen (1971), the founder of ecological economics, was the best known economist to realise that the Entropy Law imposes limits on the economic process when it is based on fossil fuels. In fact the economy is not an isolated system, it takes energy and materials from outside, and produces waste and dissipated heat. For the purposes of the analysis of the use of energy in the economy, we have no need to appeal to ‘heat death’. The evolution of an isolated system towards maximal entropy defines the so-called arrow of time as an expression of irreversibility in isolated systems. In the 19th century, thinking that the universe as a whole could be described as an isolated system, it was said that its final state would be a state of maximum entropy and zero potential for work – a state described as ‘heat death’. After a while, the system‘s potential for work becomes zero. The Entropy Law states that with every energy-based transformation a system loses part of its ability to perform useful mechanical work. In the analysis of economy-environment interactions, for example resource extraction, energy use, production, and generation of wastes, entropy is a useful concept. We cannot use these stocks again, or recycle such energy because of the Entropy Law. As they are used up, their heat content is dissipated. However, in industrial economies we are using energy, stocks of coal, oil, gas accumulated long ago. Therefore, one cannot jump from the existence of the Entropy Law to a pessimistic view regarding life and human life on Earth. The energy from the sun is the cause of photosynthesis and the source of the great wealth of life on the planet, i.e. It is a system open to the entry of energy although closed to the entry of materials. In this process, the entropy of the room has increased.Įnergy from the sun (produced by atomic fusion) reaches the Earth in very large quantities. For example, the temperature of a cup of hot coffee left in a cold room will always decrease, never increase, to eventually reach equilibrium with room temperature.

This places significant constraints on natural as well as technical processes. It states that the entropy of an isolated thermodynamic system never decreases, but strictly increases in irreversible transformations and remains constant in reversible transformations.
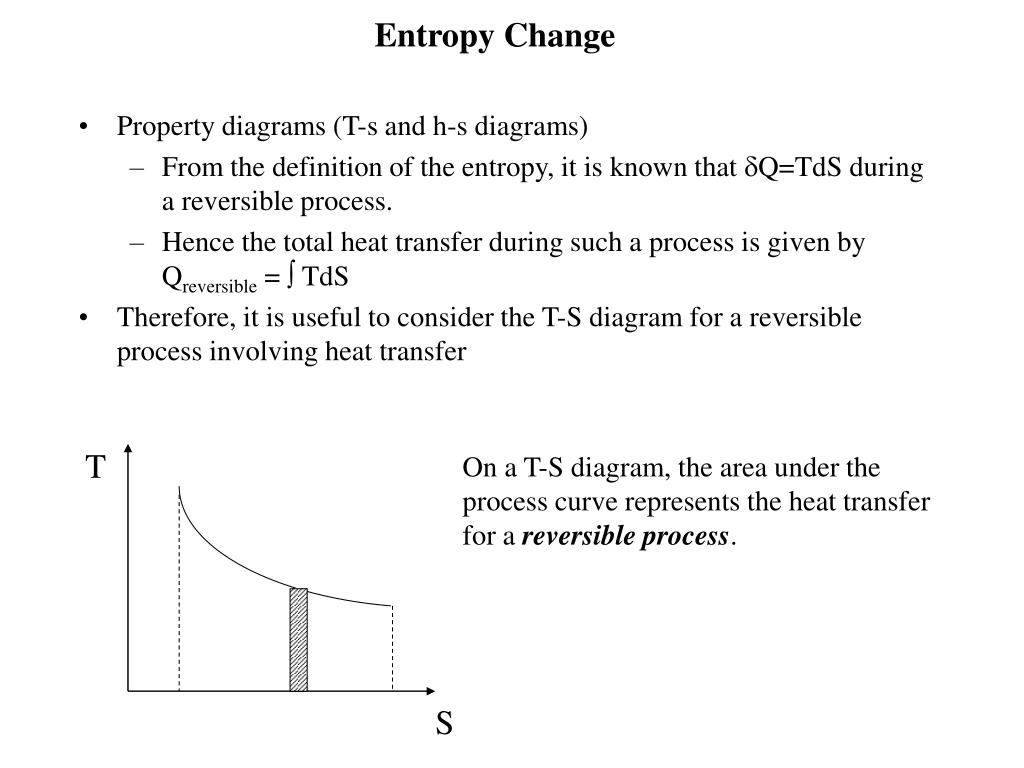
The so-called Entropy Law (the Second Law of Thermodynamics) uses this definition of entropy to express the everyday experience that transformations of energy and matter are unidirectional. Available energy corresponds to the useful part of energy, which can be transformed into work. As a piece of wood is burned, for example, its available energy – also called ’exergy’ – decreases as the wood is transformed into high entropy matter – carbon dioxide and other substances useless from an energy point of view, its original exergy dissipated as useless heat. The original notion of entropy has been applied to different contexts outside thermodynamics.Įntropy can also refer to the amount of energy available to humans. Its origins are in the 19th century when scientists like Sadi Carnot, Rudolph Clausius and Lord Kelvin wanted to understand and increase the efficiency at which steam engines performed useful mechanical work. Thermodynamics in the science of energy – the name comes from the study of how heat and movement convert into each other. The entropy concept was coined in thermodynamics to capture this fact. Examples include natural processes, such as the growing of a plant, as well as technical processes, such as the burning of fossil fuels in combustion engines. A simple way to grasp the fundamental meaning of entropy is to consider that all processes of change are irreversible.


 0 kommentar(er)
0 kommentar(er)
